BACKGROUND:
Multiple Myeloma (MM) is the 2nd most common hematologic malignancy, accounting for 32,270 new cases diagnosed in the United States each year with over 12,830 deaths and a 57% overall 5 -year survival rate. Increased incidence is seen in patients aged >60, African American (AA) race and Male population. Despite significant advancements in therapies with novel agents and immunotherapy, MM remains a challenging disease to manage due to its heterogeneity, resistance to treatment, and the risk of relapse. In this study, we aim to determine the demographics, comorbidities and trends in treatment patterns of MM patients, over time in the VA database.
METHODS:
Patients diagnosed with MM from 2000-2020 in the VA's nationwide electronic health records were identified using the VA Corporate Data Warehouse (VA CDW) and VINCI to host and analyze the data. Patients were identified using International Classification of Diseases codes for MM. Patient demographics, including drugs and therapies used, were identified through structured data in the VA CDW. We included all veterans 1) over the age of 18, 2) diagnosed with MM with 3 coded visits on different days and received 1 agent other than steroids after diagnosis date. We excluded all patients who did not have at least two VA primary care visits, or one primary care visit and one VA pharmacy refill in the one year prior to MM diagnosis. We analyzed demographic details, comorbidity burden, treatment regimens for induction and attrition rates.
RESULTS:
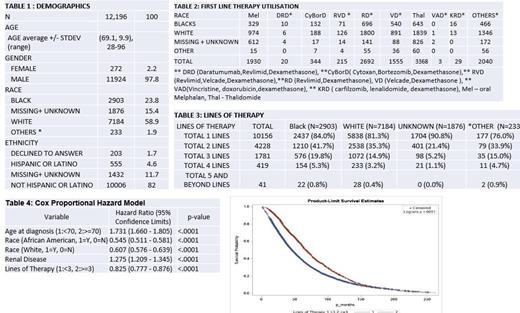
A total of 12,196 patients were identified, with a median age at diagnosis of 68 years (range, 28-96 years), of which 97.8% were males, 59% (n=7187) were White, 23% (n=2903) were Black, Other (Asian/Pacific Islanders) made up 1.9% the cohort (n=233) and for 15% cohort (n=1867), race was either unknown or missing. Comorbidities at diagnosis were the following: 4164 patients had diabetes mellitus; 1329 patients had CHF ,2159 patients had pre-existing renal disease.
10,156 patients received at least one line of therapy, with the following distribution: 215 pts - RVD, 20 - DRD, 344 - CyBORD , 1930 - oral Melphalan, 3368- Thalidomide, 2692 -Revlimid/Dexamethasone, 1555 - Velcade/Dexamethasone and 2040 patients received an induction regimen not specified in Table 2. The breakdown of first line therapy utilization in respect to race is shown in Table 2. 4228 patients received two distinct lines of therapy (LOT), 1718 patients received 3 distinct LOT and 419 patients received 4 LOT. Transitioning from 1st st to 2nd LOT, attrition rates of approximately 50.3% for Black and 56.5% for White patients were observed, and from two to three lines, rates were around 52.4% for Black and 57.8% for White patients, indicating substantial drop-offs in treatment progression.
The Cox proportional hazard model analysis reveals significant predictors for overall survival. Notably, age (70 or older) and renal disease was associated with increased mortality with hazard ratios (HR) of 1.731 and 1.275 (p < .0001 for both), respectively. Conversely, Black and White races, and receiving three or more therapy lines, was associated with increased OS, with HRs of 0.545, 0.607, and 0.825 (p < .0001 for all).
CONCLUSIONS:
This comprehensive overview of veterans with MM in the VA system over a 20-year period reveals heterogeneity in treatment utilization as expected with the changing landscape of MM treatment. A detailed analysis on the nature of subsequent lines of therapies, including stem cell transplant utilization, access to clinical trials is ongoing and will be submitted as a full manuscript publication. Additionally, the data on the staging and cytogenetics is also being obtained.
Disclosures
Kaur:Cellectar: Consultancy; Janssen: Consultancy, Research Funding; Pfizer: Consultancy; BMS: Consultancy, Research Funding; Arcellx: Consultancy, Research Funding; Abbvie: Research Funding; Kedrion: Consultancy; Sanofi: Consultancy. Anderson:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Cellectar: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Beigene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Prothena: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.